Is Copper Sulfate an Element Compound or Mixture
Hydrated copper sulfate crystals CuSO45H2Os from copper sulfate solution CuSO4aq. Describe how to produce pure dry crystals of copper sulfate from a copper sulfate solution.
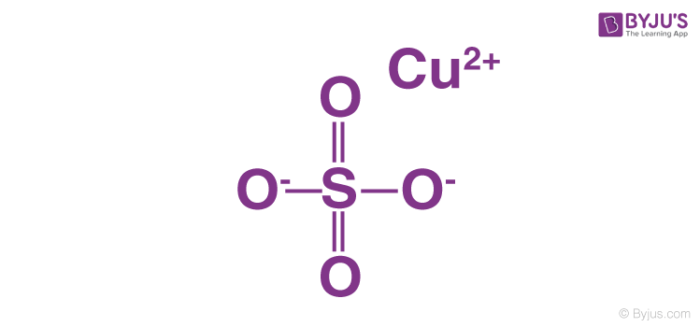
Copper Sulfate Structure Properties And Uses Of Cuso4
Water is a compound because it contains two different atoms hydrogen and oxygen chemically bonded together.
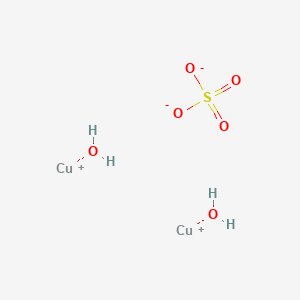
. It is therefore. You can classify matter into element compound or mixture according to its composition. An atom A beaker containing a black-yellow powder has a magnet put into it.
Understand that a pure substance has a fixed melting and boiling point but that a mixture may melt or boil over a range of temperatures. Salt Sodium Chloride Compound. Classify the following as either elements compounds homogeneous mixtures solutions or heterogeneous mixtures.
Gently heat the solution. By definition a mixture consists of two or more elements that have been physically combined in any proportion. Introduction Properties Compounds Sources and Ores.
Copper sulfate can be broken down into simpler substances by chemical means. Learn vocabulary terms and more with flashcards games and other study tools. A Chestnut compound is a mixture of copper sulfate and ammonium carbonate used in horticulture that prevent damping off in seedlings.
A copper II sulfate b Kool. Copper sulphate is a compound not an element and therefore has a formula not a symbol. Its obviously an element as it is made from only one type of atoms.
Classify the following as either elements compounds homogeneous mixtures solutions or heterogeneous mixtures. An element is a standalone matter. Copper II compounds of commercial value include cupric oxide CuO cupric chloride CuCl2 and cupric sulfate CuSO4.
Secondly what compound is copper in. Copper sulfate CuSO4 Compound Air Homogeneous mixture solution Oil water Heterogeneous mixture Sulfur S Element Raisin bran Heterogeneous mixture Soil Heterogeneous mixture Sodium bicarbonate NaHCO3 Compound Iron Fe Element Mustard Homogeneous mixture suspension Sets with similar terms homogeneous and heterogeneous. Whether a compound is a mixture of different type of atoms of different elements.
Study the diagrams representing elements compounds and mixtures shown and choose the correct statement from the following. Unhydrated copper II sulfate has the formula CuSO4. Sections in this article.
Element Carbon Dioxoide compound water compound Calcium Carbonate compound Copper Sulfate compound Sodium Bicarbonate compound Sodium Chloride compound Sodium. The most important chemical compound of copper is copper sulfate pentahydrate also called bluestone or blue vitriol. Gently heat the copper sulfate solution to evaporate the solvent.
A copper II sulfate b Kool Aid c wood d plastic e lined paper f gadolinium. Some examples of elements are calcium magnesium iron copper carbon and helium. Verdigris is basic copper acetate.
Other compounds include Paris green Bordeaux mixture a cyanide a chloride oxides and a basic carbonate. Solve for the formula mass of NH42CO3 or ammonium carbonate. Start studying Element Compound or Mixture.
Understand how to classify a substance as an element compound or mixture. The remaining solution is cooled to allow the crystals to form. But they can combine with other elements to form compound or mixture.
The manufacturing process of brass is simply the mixture of two molten elements copper and zinc in any proportion whatsoever. To gain further clarification lets look at several differences between elements mixtures and compounds. When two or more elements combine together.

Get Examples Of Pure Substances Pure Products Substances Chemistry Experiments
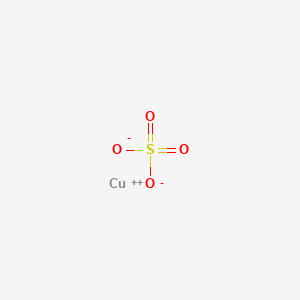
No comments for "Is Copper Sulfate an Element Compound or Mixture"
Post a Comment